REGENECORE is the world's first IL4Rα-IL5 double antibody completed the first subject administration
- Categories:Company news
- Author:
- Origin:
- Time of issue:2023-07-07
- Views:0
REGENECORE is the world's first IL4Rα-IL5 double antibody completed the first subject administration
(Summary description)REGENECORE antibody drugs development enter the clinical research stage
- Categories:Company news
- Author:
- Origin:
- Time of issue:2023-07-07
- Views:0
On July 4, 2023, Nanjing RegeneCore Biotech Co., Ltd. successfully administered the first dose of its self-developed recombinant anti-human IL-4Rα/IL-5 bispecific antibody injection (RC1416 injection) to a volunteer at China-Japan Friendship Hospital. This milestone marks Nanjing RegeneCore's entry into the clinical research phase for antibody drug development. The study aims to evaluate the safety and tolerability of RC1416 in Chinese healthy subjects.
RC1416 is Nanjing RegeneCore's first bispecific antibody drug developed based on sdAb technology. By blocking the IL-4, IL-5, and IL-13 signaling pathways, it holds potential for treating diseases associated with overactivation of the Th2-type immune response.
Nanjing RegeneCore continues to advance technology innovation driven by clinical needs and market demand, steadfastly progressing on its mission to "safeguard human health with exceptional medicines."
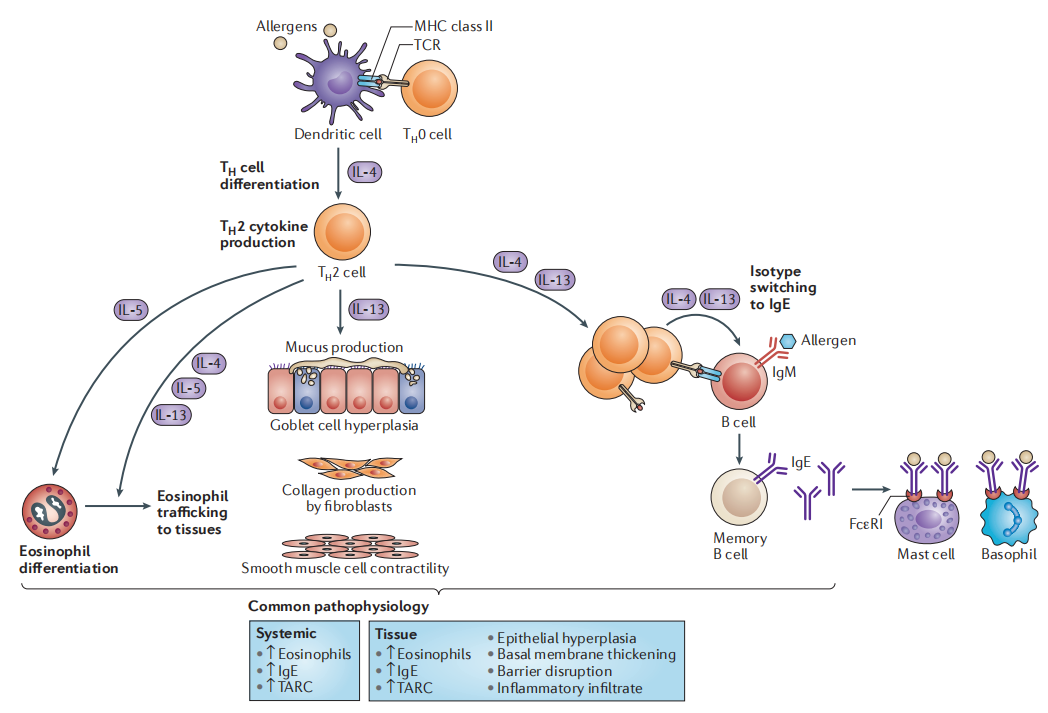
Figure 1: Schematic diagram of molecular mechanisms related to Th2 inflammation[1]
Reference:
[1]Gandhi NA, Bennett BL, Graham NM, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016 Jan;15(1):35-50. doi: 10.1038/nrd4624. Epub 2015 Oct 16. PMID: 26471366.Reference:
Scan the QR code to read on your phone
TEL:
Address: Room 07 Building 16 Treehouse, No. 73, Tanmi Road, Jiangbei New District, Nanjing
Enterprise email:rjk@regenecore.com

WeChat cooperative consultation
You are the th visitor

 025-58608860
025-58608860 

 Contact
Contact 